Pharma exports hit $30.47bn with 9.4% growth; the government and industry target double-digit rise and biopharma push.
India’s pharmaceutical exports have crossed the USD 30 billion milestone, with the government and industry jointly setting sights on accelerated growth and deeper global market penetration. An industry interaction under the Chintan Shivir series, held in Ahmedabad, focused on “Scaling up Pharma Exports” and underscored the Centre’s push to expand India’s export footprint through closer coordination with regulators and industry, in line with the vision of Prime Minister Narendra Modi.
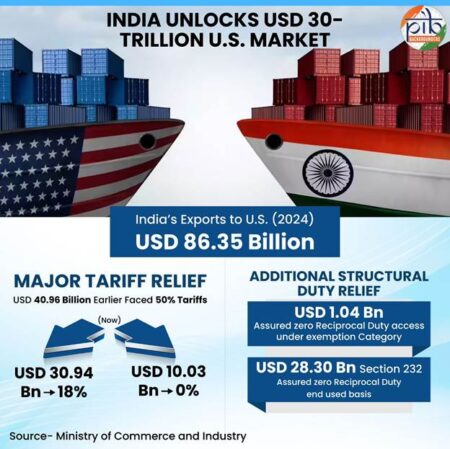
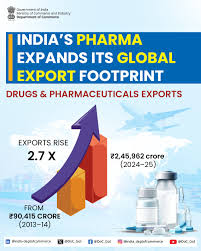
Pharmaceutical exports stood at USD 30.47 billion in FY 2024–25, registering a 9.4% year-on-year growth. The sector, currently valued at around USD 60 billion, is projected to reach USD 130 billion by 2030. India ranks third globally by volume, exporting medicines to more than 200 markets, with over 60% of shipments going to highly regulated destinations. The United States accounts for 34% of exports, while Europe contributes 19%. Industry participants indicated readiness to target double-digit export growth in 2026–27.
The programme opened with a video address from the Commerce Secretary, who stressed sustained engagement with exporters and swift resolution of challenges in regulated markets. The message reiterated India’s commitment to strengthening its reputation as a trusted global supplier of affordable, high-quality medicines.
Senior officials from the Department of Commerce, Directorate General of Foreign Trade, Central Drugs Standard Control Organisation and state drug authorities joined industry stakeholders to deliberate on regulatory processes, export facilitation and policy alignment—particularly for MSMEs facing compliance and inspection hurdles.
Discussions also reflected priorities outlined in the Union Budget 2026–27, which places biopharma and biologics at the core of India’s future healthcare strategy. The proposed Biopharma SHAKTI initiative, with an outlay of ₹10,000 crore over five years, aims to build end-to-end domestic capabilities in biologics and biosimilars, reduce import dependence and enhance India’s competitiveness in global supply chains, with a target of capturing 5% of the global biopharma market.
Participants reviewed proposals to establish three new National Institutes of Pharmaceutical Education and Research, upgrade seven existing NIPERs, develop over 1,000 accredited clinical trial sites and strengthen CDSCO capacity through specialised scientific manpower—steps aimed at faster evaluation of complex products and higher regulatory confidence.
Panel discussions traced the export journey from manufacturing excellence to global market acceptance, focusing on quality systems, compliance readiness and moving up the value chain. Exporters were also briefed on opportunities emerging from trade engagements with the European Union and the United States, including access to the EU’s USD 572.3 billion pharma and medical devices market.
A presentation by the Pharmaceuticals Export Promotion Council of India (PHARMEXCIL) and updates on the upcoming iPHEX exhibition followed, with around 200 exporters, mainly from western India, participating in the interaction.
The Ahmedabad deliberations reinforced a clear direction: faster and predictable approvals, stronger regulatory cooperation and a strategic shift from volume-led exports to higher-value segments such as biologics, biosimilars and innovation-driven products, as India positions itself for the next phase of global pharmaceutical leadership.
Source: PIB